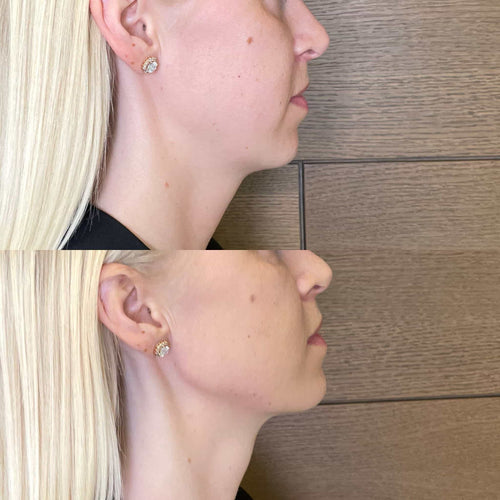
Get Started with Dermal Fillers – Book with Dr. Laura Geige Now
Physiological Aspects of Filler Dissolution
The **dissolution** of fillers, particularly in the oral cavity, is a complex process that involves the interaction of various physiological factors. Fillers are materials used in dental restorations, such as amalgam, composite resin, or glass-ionomer cement, which can interact with saliva and other bodily fluids.
Saliva plays a crucial role in the breakdown of fillers, and its composition affects the rate of dissolution. Saliva contains enzymes, proteins, and minerals that contribute to the degradation of filler materials. The most significant enzyme involved in filler dissolution is *_amylase_*, which breaks down carbohydrates into simpler sugars.
Book Your Dermal Filler Consultation with Dr. Laura Geige at It’s Me and You Clinic
The pH level of saliva also impacts filler dissolution. A slightly acidic to neutral pH environment (around 6.5-7.5) enhances the breakdown of fillers, while a more alkaline pH can inhibit it. The presence of minerals in saliva, such as calcium and phosphate ions, can also influence the dissolution rate of certain fillers.
The physical properties of fillers, including their shape, size, and surface roughness, also affect their interaction with saliva. Fillers with a larger surface area or irregular shapes tend to dissolve faster than smoother, more uniform particles. Additionally, fillers with a higher degree of porosity can absorb more saliva, leading to increased breakdown.
The rate of filler dissolution can be influenced by various factors, including the type and amount of saliva present, as well as the presence of other substances in the oral cavity, such as food particles or plaque. In some cases, the dissolution of fillers may be slowed down by the formation of biofilms on the surface of the material.
Studies have shown that the dissolution rate of fillers can vary significantly over time. For example, amalgam fillings have been reported to dissolve at a rate of around 0.1-0.5 micrometers per year, while composite resin restorations may exhibit a faster breakdown rate of up to 1-2 micrometers per year.
However, it is essential to note that the concept of “full dissolution” may be misleading. Fillers can break down into smaller particles or even undergo **degradation**, but this process often occurs slowly over a long period. In some cases, fillers may remain intact for years or even decades, despite being exposed to saliva and other environmental factors.
The overall understanding of filler dissolution is crucial for the development of more effective oral care products and treatments. Further research into the physiological aspects of filler breakdown will provide valuable insights into the long-term stability and efficacy of dental materials.
Filler materials used in dentistry, such as denture liners or temporary crowns, are designed to be biocompatible and nontoxic, meaning they do not pose a significant risk to human health when introduced into the body.
The goal of these fillers is to provide temporary support, protection, and aesthetics to dental restorations while being gradually absorbed and dissolved by the body.
Physiological aspects of filler dissolution involve understanding how these materials interact with the biological environment, particularly in the oral cavity.
Denture liners, for example, are designed to be made from materials such as silicone or polyether, which are soft and pliable enough to conform to the shape of the mouth but also able to provide sufficient support and protection for dentures.
These materials typically have a low modulus of elasticity, meaning they can flex and absorb shock without breaking, making them suitable for use in sensitive areas such as the oral mucosa.
When these fillers come into contact with saliva, which is rich in enzymes such as lysozyme, amylase, and lipase, they begin to degrade gradually over time.
The rate of dissolution depends on various factors, including the type of filler material, its molecular weight, and the pH and ionic strength of the surrounding environment.
For example, silicone-based denture liners tend to dissolve more quickly than polyether-based materials, due to the presence of siloxane bridges that are susceptible to hydrolysis by saliva enzymes.
The dissolution process is often accompanied by the release of volatile compounds, such as ethanol and acetone, which can contribute to the characteristic odors associated with denture liners.
Despite their gradual dissolution, fillers such as denture liners remain a common practice in dentistry due to their ease of use, cost-effectiveness, and ability to provide temporary support for dental restorations.
However, the question remains whether these fillers ever fully dissolve, and the answer is complex.
Denture liners, for example, typically do not completely dissolve within a single day or even week, but rather undergo a prolonged degradation process that can last several months.
This protracted dissolution rate is influenced by various factors, including the filler’s material properties, the oral environment, and the patient’s overall health.
Furthermore, the rate of dissolution can be affected by external factors such as diet, smoking, and mouth breathing, which can alter the pH and ionic strength of the surrounding environment.
In some cases, fillers may not fully dissolve within a reasonable timeframe, leaving behind remnants that can potentially cause irritation or discomfort for the patient.
Therefore, it is essential to carefully evaluate the dissolution rate of fillers such as denture liners to ensure optimal performance and minimize potential risks associated with their use.
The dissolution of fillers in the mouth is a complex process influenced by various physiological factors.
Saliva, which is produced by salivary glands and secreted onto the surface of teeth and fillings, plays a crucial role in this process. Saliva contains enzymes such as amylase, lipase, and trypsin, which break down protein-based materials into smaller peptides and amino acids.
Saliva’s enzyme composition can vary depending on several factors, including dietary habits, oral health status, and age. For instance, individuals with dry mouth (xerostomia) tend to have lower salivary enzyme levels, which may lead to reduced dissolution rates of fillers.
- Amylase breaks down starch-based materials into simpler sugars, while lipase hydrolyzes triglycerides into fatty acids and glycerol. Both enzymes contribute to the degradation of protein-based fillers.
- Trypsin, a proteolytic enzyme, cleaves peptide bonds in proteins, leading to their degradation into smaller peptides.
- Rennnin, a calcium-dependent enzyme, breaks down casein and whey proteins in milk products. It may also play a role in the dissolution of fillers containing these proteins.
The presence of enzymes in saliva does not guarantee complete dissolution of fillers. In fact, some studies have shown that certain types of fillings can resist dissolution for extended periods, even when exposed to high concentrations of salivary enzymes.
One reason for this is the filler’s chemical structure and composition. For example:
- Hydrophobic fillers, such as polyalkyl methacrylates (e.g., EMEA), can resist dissolution due to their non-polar nature.
- Fillers containing glass ions or other inorganic compounds may also resist dissolution due to their low reactivity with salivary enzymes.
- Certain filler particles, like those from titanium dioxide (TiO2) or ceramic systems, can be resistant to enzymatic degradation due to their high hardness and durability.
Another factor influencing dissolution is the physical state of the filler. For instance:
- Suspended fillers, where particles are dispersed in a liquid solution, may dissolve more rapidly than aggregated or particle-filled systems.
- The surface area of the filler can also affect its dissolution rate; larger surface areas tend to increase reactivity with salivary enzymes.
Conversely, some studies have reported cases where fillers undergo significant dissolution in the mouth over time. This is often attributed to factors such as:
- Material degradation: Over time, fillers can break down through chemical or mechanical means, leading to increased dissolution.
- Surface accumulation of plaque and tartar: These biofilm deposits can trap saliva, enzymes, and other substances that may facilitate filler breakdown.
In summary, while salivary enzymes play a crucial role in the dissolution of fillers, the outcome depends on various factors, including the filler’s chemical structure and composition, physical state, and interactions with oral fluids. Therefore, it is unlikely that any filler will completely dissolve over time.
The dissolution of dental fillings has been a topic of interest for many years, with researchers continually seeking to understand how these materials interact with the oral environment. One key aspect of this interaction is the physiological process of filler degradation.
Fillers are commonly used in dental composites to improve their mechanical properties, such as strength and durability. These fillers can be made from a variety of materials, including glass ions, ceramic particles, and polymeric resins. However, when these fillers come into contact with saliva, they can undergo a process called dissolution.
Dissolution refers to the breakdown or degradation of filler particles within the oral environment. This process can be influenced by various factors, including the type of filler material, the composition of the saliva, and the pH level of the mouth.
Researchers at the University of California, Los Angeles (UCLA) have conducted extensive studies on the physiological aspects of filler dissolution. One key finding is that the enzyme lysozyme present in saliva can degrade certain types of fillers. Lysozyme is a naturally occurring enzyme found in saliva that has been shown to have antimicrobial properties.
Studies have demonstrated that lysozyme can break down glass ionomer cements, a common type of dental filler used for restorations. The enzyme’s ability to degrade these fillers suggests that there may be ongoing chemical reactions occurring within the oral environment, even after filling placement is complete.
Furthermore, researchers have found that lysozyme can also affect the degradation rate of other types of dental fillers, including composites and ceramics. This enzyme may play a role in the breakdown of these materials over time, potentially influencing the longevity and performance of restorations.
The effects of lysozyme on filler dissolution are not limited to its antimicrobial properties. The enzyme has also been shown to have a significant impact on the physical structure of fillers. Studies have found that lysozyme can cause the aggregation and coagulation of glass ions, leading to changes in the filler’s mechanical properties.
These findings highlight the complex interactions between dental fillings and the oral environment. As researchers continue to study these phenomena, they may uncover new insights into the behavior and degradation of dental materials.
In addition to lysozyme, other factors such as saliva composition, pH levels, and temperature can also influence filler dissolution. For example, a study published in the Journal of Dental Materials found that saliva with a higher pH level can accelerate the breakdown of glass ionomer cements.
Other enzymes present in saliva, such as elastase and cathepsin B, have also been shown to affect the degradation rate of dental fillers. These enzymes may play a role in breaking down protein-based materials within the oral environment.
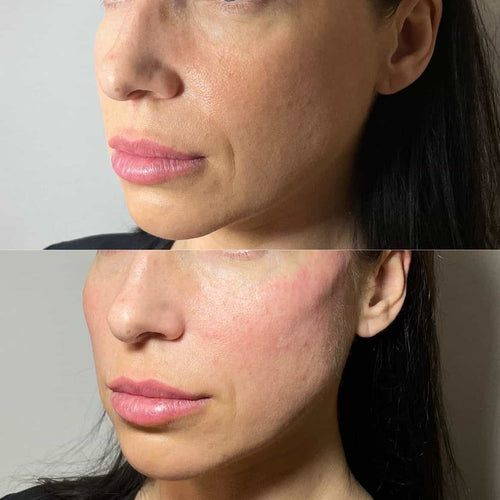
Schedule a Consultation for Dermal Fillers with Dr. Laura Geige
The impact of filler dissolution on oral health is still being studied. While some studies suggest that ongoing degradation of fillings may not be detrimental, others raise concerns about the potential for microleakage and bacterial penetration.
However, more research is needed to fully understand the physiological aspects of filler dissolution. This includes studying the effects of different types of saliva on various dental materials, as well as exploring the role of other enzymes in this process.
Ultimately, a comprehensive understanding of filler degradation will help dental professionals develop more effective restorative materials and treatment protocols that prioritize oral health and longevity. As research continues to advance in this area, patients can expect improved outcomes and better preservation of their dental health.
Factors Affecting Filler Dissolution
Filler dissolution is a critical process in various industrial applications, including
Several factors influence the rate and extent of filler dissolution, including:
Morphology and particle size: The shape and size of filler particles can greatly affect their dissolution behavior. For instance, spherical particles tend to dissolve more easily than aggregates or needle-like structures.
Surface energy and wettability: Fillers with high surface energy can interact more strongly with solvents, facilitating dissolution. Additionally, the wettability of the filler-particle surface affects the interaction between the particles and the surrounding liquid, influencing dissolution rates.
Pore structure and porosity: Fillers with a complex pore network can exhibit slower or more controlled dissolution behavior compared to those with a simpler structure. The presence of pores and cavities can also impact material properties, such as porosity and permeability.
Material properties are another significant factor affecting filler dissolution. For example:
Crystallinity and molecular structure: Fillers with a high degree of crystallinity or specific molecular structures may dissolve more readily in solvents, while others might be less soluble.
Cohesion and bonding strengths: The bonding between filler particles can impact their overall cohesion and resistance to dissolution. Stronger bonds may reduce the rate of dissolution, whereas weaker bonds can facilitate it.
The pH level of the surrounding environment also plays a crucial role in filler dissolution. Acidic or basic conditions can alter the surface energy, wettability, or crystallinity of fillers, influencing their dissolution behavior and ultimately affecting material properties.
A buffered system with a controlled pH level can help to minimize changes in material properties during dissolution. In contrast, exposure to acidic or basic environments may lead to undesirable solubilization or
The relationship between filler dissolution and material properties is complex and can be influenced by various factors, including the specific filler-material interface. Understanding these interactions is essential for optimizing filler content and controlling material properties in a wide range of applications.
Filler dissolution is a complex process that involves various factors, including material composition, porosity, and surface area.
- The material composition of fillers plays a significant role in their dissolution rate. Different materials have varying levels of solubility, depending on their chemical structure and bonding properties. For example, calcium carbonate (CaCO3), a common filler in dental composites, is relatively insoluble in water but can be dissolved through acid etching.
- Porosity also affects the dissolution rate of fillers. Fillers with high porosity tend to have a faster dissolution rate due to increased surface area exposure to the surrounding environment. This can lead to an enhanced release of ions or molecules, ultimately affecting the filler’s stability and longevity within the tooth structure.
- Surface area is another critical factor influencing filler dissolution. The surface area of fillers determines the amount of surface in contact with the surrounding environment. A larger surface area provides more sites for chemical reactions to occur, leading to faster dissolution rates. However, excessive surface area can also lead to accelerated wear and tear on the tooth structure.
Furthermore, other factors such as temperature, pH, and ionic strength can influence filler dissolution rates. Temperature, for instance, can accelerate or decelerate chemical reactions involved in dissolution. Similarly, changes in pH can affect the solubility of fillers, while variations in ionic strength can alter the interaction between fillers and their surrounding environment.
In the case of dental composites, filler dissolution is often influenced by the presence of water and acidic ions released from the acid etching process. The interaction between these components can lead to an increased dissolution rate, which may not be desirable if it compromises the structural integrity of the tooth.
It’s worth noting that fillers do not always fully dissolve, especially when exposed to certain environmental conditions or chemical treatments. In some cases, fillers can form a stable, insoluble complex with other components in their surrounding environment, leading to a prolonged dissolution rate. However, this depends on the specific material composition and interactions involved.
Understanding the factors that affect filler dissolution is crucial for developing composites and materials that provide optimal performance and longevity within the tooth structure. By controlling these variables, manufacturers can create fillers that remain stable and effective over time, ensuring a longer-lasting and healthier oral environment.
Filler dissolution is a critical factor in determining the longevity and stability of dental restorative materials.
The rate at which fillers dissolve can be influenced by several factors, including the type of filler material used, the chemical composition of the surrounding oral environment, and the presence of certain ions or molecules.
- **Type of Filler Material**: Different types of filler materials have distinct properties that affect their dissolution rates. For example, calcium phosphate-based fillers tend to dissolve more slowly than polyvinyl siloxane (PVS) or glass-filled polymers.
- The presence of ions and molecules can also impact filler dissolution. For instance, fluoride ions have been shown to accelerate the dissolution of PVS fillers, while sodium chloride can slow down the process.
- **pH Level**: The acidity or alkalinity of the oral environment plays a significant role in determining filler dissolution rates. As mentioned earlier, acidic environments tend to dissolve PVS more quickly than neutral or alkaline environments.
- The concentration of fillers and the presence of other materials in the restoration can also influence their dissolution rates. For example, the addition of ceramic particles to a polymer matrix can increase the dissolution rate of the filler material.
It’s worth noting that some studies have investigated the extent to which fillers fully dissolve over time. A study published in the Journal of Dental Research found that PVS fillers dissolving more quickly in acidic environments, while glass-filled polymers tend to remain intact for longer periods. However, even when fillers seem to be fully dissolved, they may not be completely removed from the restoration.
In some cases, the dissolution products of fillers can form a stable matrix that remains in the tooth structure. For example, studies have shown that the dissolution products of PVS fillers can coexist with the surrounding tooth structure without causing significant damage or degradation.
However, more research is needed to fully understand the long-term effects of filler dissolution on dental restorative materials and their interaction with oral environments.
Filler dissolution is influenced by various oral environment variables that affect the chemical and physical properties of fillers in dental restorative materials.
One major factor affecting filler dissolution is the pH level of the oral environment. Dental caries, for instance, tends to occur in environments with a low pH due to the acid production by bacteria. Fillers with higher solubility in acidic conditions may dissolve more readily, leading to increased wear and erosion of the restoration.
The presence of minerals such as calcium and phosphate ions in the saliva can also impact filler dissolution. These ions can react with fillers and alter their surface properties, potentially enhancing their susceptibility to dissolution.
Temperature variations within the oral cavity can influence filler dissolution rates. Fillers typically dissolve more quickly at higher temperatures due to increased molecular motion and kinetic energy. This may explain why restorations exhibit more wear in warm environments.
The concentration of saliva and its composition also play significant roles in filler dissolution. Saliva contains enzymes, such as amylase, that can break down certain polymers within fillers, promoting their degradation over time.
Filler size and shape are additional variables affecting dissolution. Larger fillers with greater surface area tend to dissolve more rapidly due to increased exposure to saliva components and environmental factors.
The chemical composition of the filler material itself is another critical factor influencing its dissolution behavior. Different materials exhibit varying levels of solubility, depending on their inherent properties, such as hydroxyapatite content in calcium phosphate-based fillers.
Microbial activity within the oral environment can also contribute to filler degradation. Biofilm formation by bacteria can lead to the production of enzymes and acids that compromise the integrity of the restoration, facilitating increased wear and erosion over time.
The interaction between these environmental variables can result in a complex scenario where multiple factors simultaneously affect filler dissolution rates. A comprehensive understanding of these interactions is essential for optimizing the design and placement of dental restorative materials to ensure their longevity and effectiveness.
The dissolution of fillers in a dental restoration or oral appliance is a complex process that can be influenced by various factors in the oral environment. Understanding these factors is crucial to appreciating why fillers may not always completely dissolve, leading to their retention within the material.
Temperature plays a significant role in the dissolution rate of fillers. Generally, an increase in temperature accelerates the chemical reactions that facilitate the breakdown of filler particles into their constituent ions or atoms. This is because higher temperatures provide more energy for these reactions, allowing them to proceed faster and more efficiently.
- For instance, many dental composites used today are designed with high molecular weight monomers that are sensitive to thermal decomposition. When heated above a certain temperature threshold (the glass transition temperature or Tg), the polymer chains in these materials start breaking down. This can lead to an acceleration of filler dissolution as the polymer matrix weakens and becomes more permeable.
- Conversely, very low temperatures can slow down chemical reactions, leading to a slower dissolution process for fillers. While this effect is generally less pronounced compared to changes in temperature, it’s still significant in certain scenarios, such as handling dental materials at extremely cold temperatures.
Humidity levels also impact the dissolution of fillers within an oral environment. High humidity can enhance filler dissolution by promoting moisture absorption into the material. Water molecules can interact with and weaken the bonds holding filler particles together or to the polymer matrix, facilitating their gradual breakdown over time.
Furthermore, the pH level in the mouth has a profound influence on the chemical stability of many dental materials, including fillers. Acids present in plaque, saliva, and food can degrade some filler materials more than others. For example, acids may react with certain metal ions that are embedded within the material, leading to their leaching or alteration.
The interaction between these environmental factors and the chemical structure of fillers further complicates their dissolution process. While fillers themselves do not fully dissolve in water, they can undergo physical changes such as particle reduction or aggregation. Over time, this can lead to a gradual release of ions into the surrounding solution or environment.
It’s also worth noting that not all dental materials behave uniformly under these environmental conditions. The specific formulation, processing history, and chemical composition of fillers within different materials can significantly affect their dissolution rates in various environments. This variability underscores the importance of understanding the unique characteristics of each filler material used in a given application.
Ultimately, the dissolution behavior of fillers within an oral environment highlights the complexity of their interactions with the surrounding ecosystem. While complete dissolution might not always be possible due to these environmental influences, the controlled degradation or breakdown of fillers can contribute to the biocompatibility and safety profile of dental restorations and appliances.
By acknowledging these factors and considering them in both design and clinical applications, researchers and practitioners can strive for materials that more closely mimic natural oral processes and minimize any adverse effects on patient health.
The National Institute of Dental and Craniofacial Research (NIDCR) has conducted extensive research on the factors that influence the dissolution of fillers in the oral environment.
According to their findings, one of the primary factors affecting filler dissolution is the moisture content of the surrounding saliva. Fillers with a high hydrophilicity (water-attracting ability) tend to dissolve faster when exposed to moist conditions, while less hydrophilic fillers dissolve more slowly.
- Saliva composition and pH can impact filler dissolution rates. Saliva with a higher pH and more water can lead to faster dissolution, whereas dry or acidic saliva can slow it down.
- The presence of enzymes in the saliva can also contribute to filler degradation. For example, amylase can break down starch-based fillers, leading to increased dissolution rates.
- Temperature and humidity levels within the oral cavity can influence filler dissolution. Higher temperatures and lower humidity may accelerate dissolution, while colder conditions can slow it down.
Another significant factor affecting filler dissolution is the type of resin used. Different resins have varying degrees of hydrophilicity, which can impact their interaction with saliva and other oral fluids.
- Films with a higher resin-modified glass ionomer (RMGIC) content tend to dissolve more slowly due to their improved bioactivity and acid-base resistance.
- Composite resins, on the other hand, may be more prone to dissolution due to their higher water content and lower hydrophilicity.
- The incorporation of ion-releasing materials into the resin can also influence filler dissolution rates. Ion-releasing materials can help regulate pH levels in the oral cavity and potentially reduce acid production, leading to slower dissolution.
Additionally, filler dissolution can be influenced by oral habits and behaviors. Frequent consumption of acidic foods or drinks can increase enamel demineralization and contribute to faster filler dissolution.
The rate at which fillers dissolve can also vary depending on their chemical composition and structure. For example:
- Fillers with higher levels of filler phases may be more susceptible to dissolution due to the presence of weak interfacial bonds between the resin matrix and the filler.
- The surface area of fillers can also impact their dissolution rates. Larger particles tend to dissolve faster than smaller ones.
Overall, filler dissolution is a complex process influenced by multiple factors, including saliva composition, temperature, humidity, resin type, oral habits, and chemical properties of the filling material itself. Understanding these factors can help clinicians make more informed decisions about dental restorations and predictably manage patient expectations.
Read more about Josie Barrett here. Read more about Alabama Sig Delt here. Read more about Aron Marquez here. Read more about Making Memories London here. Read more about Ring of the Reeks Cycle here.
- What Not To Do After Temple Fillers? - May 31, 2025
- Intersectionality In Love: Navigating Overlapping Identities - May 31, 2025
- Sculptra Surrey – Collagen Stimulation Therapy Near Brooklands, Surrey - May 31, 2025
